Objectives and follow-ups
Objectives
The epidemiological EGEA cohort (Epidemiological study on the Genetics and Environment of Asthma, bronchial hyperresponsiveness and atopy) was initiated in 1991 to meet the following general objectives:
- to identify the genetic factors of asthma,
- to identify the environmental factors of asthma and
- to clarify the clinical heterogeneity of asthma, i.e. the different forms in which this disease manifests itself.
A better understanding of the relationships between asthma and environment, lifestyle and genetics is essential to develop preventive measures and to improve asthma management. The EGEA study provides public health information with the objective of improving the respiratory health of the population.
The EGEA cohort was set up in 5 French cities: Paris, Lyon, Grenoble, Montpellier and Marseille, at the initiative of research teams from Inserm (French National Institute for Health and Medical Research). Since 2013, the cohort is coordinated by a research team in environmental epidemiology at Inserm in Grenoble. This research project is carried out with many national and international scientific partners.
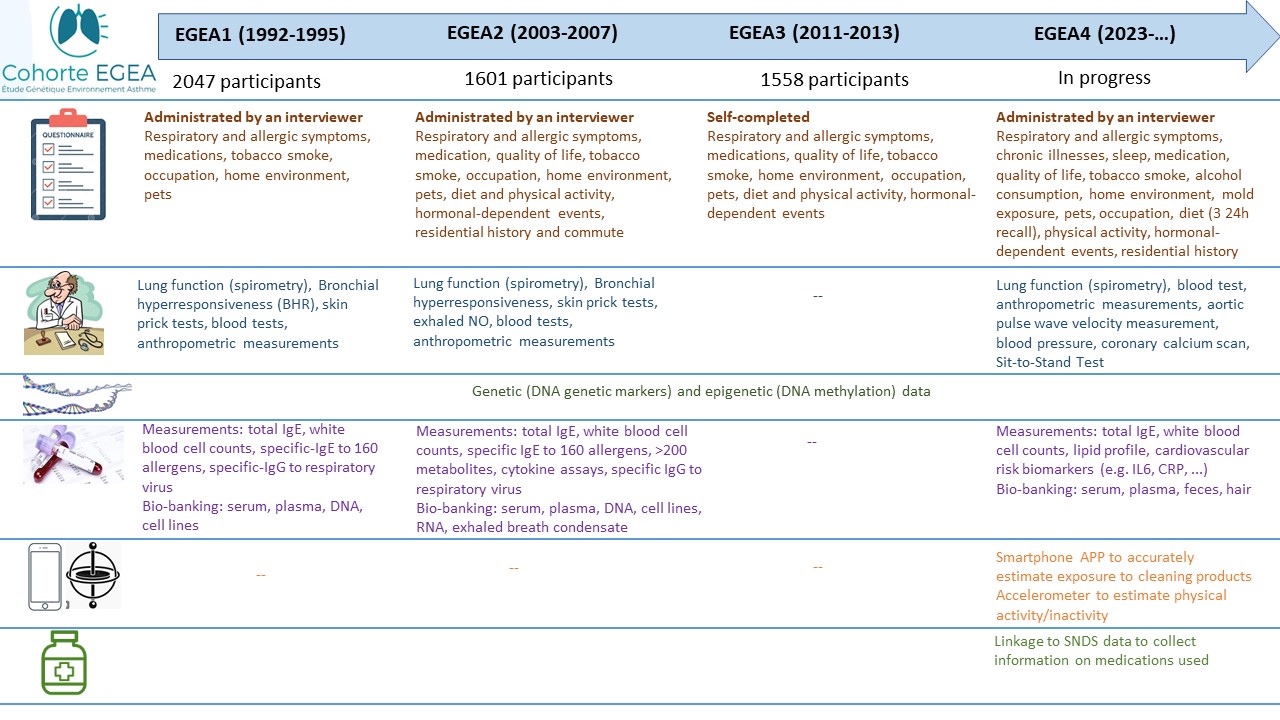
To meet the research objectives, 388 children and adults who had asthma were recruited in one of the 8 chest clinics from 5 cities participating to the study (Paris (Cochin, Necker, Bichat, Trousseau), Lyon, Marseille, Montpellier, Grenoble), then their first-degree relatives (parents and siblings for participants recruited as children or spouses and offspring for participants recruited as adults) and 415 children and adults from the general population ("controls") were included. A total of 2047 participants were first examined between 1992 and 1995. Approximately 12 years later (2003-2007) the study participants were invited to participate in a first follow-up and again approximately 7 years later (2011-2013). A new follow-up of the study is being set up and EGEA participants will be invited in 2023-2026, 30 years after their recruitment in the cohort. The EGEA cohort will then be one of the few cohorts in the world with such a long-term follow-up.
The EGEA study, with the family design, allows the study of the genetics of asthma.
In order to estimate the genetic effects, 348 families were selected from one asthmatic case. An additional 40 families with two asthmatic siblings were also included to allow for specific genetic studies. The genetic data of all EGEA participants allows to locate and identify the genes predisposing to asthma.
The EGEA study, with the family design, allows the study of the genetics of asthma.
In order to estimate the genetic effects, 348 families were selected from one asthmatic case. An additional 40 families with two asthmatic siblings were also included to allow for specific genetic studies. The genetic data of all EGEA participants allows to locate and identify the genes predisposing to asthma.
The main environmental factors studied in the EGEA cohort are:
- Tobacco smoke: active smoking (start of smoking, stop of smoking, duration of smoking, ...) and passive smoking
- The home and work environment: Including exposure to cleaning products and occupational exposure to asthmogens (which cause or exacerbate asthma)
- Nutrition / physical activity: In particular, the evaluation of the impact of diet on asthma
- Temperature
- Air pollution: especially the particles in the air we breathe
- Indoor environment: including exposure to mould
The main health outcomes studied in the EGEA cohort are:
- Asthma and different types of asthma (e.g. asthma control, asthma severity)
- Respiratory symptoms (wheezing, shortness of breath, cough, sputum, etc.) through a detailed respiratory health questionnaire
- Rhinitis (hay fever) and nasal symptoms
- Eczema and associated skin symptoms
- Lung function parameters obtained by spirometry (with metacholine test)
- Allergic sensitization: skin prick tests
- Biological parameters: Total IgE, eosinophils, neutrophils, immune assays, etc.
- Quality of life
- Cardiovascular health measures (at EGEA4 with a measurement of arterial stiffness by pulse wave velocity measurement and coronary calcium scan measurement by thoracic scanner)
The EGEA cohort includes a total of 2120 asthmatic and non-asthmatic participants.
A first survey (EGEA1) took place between 1991 and 1995.
A second survey (EGEA2) was conducted between 2003 and 2007. In this second survey, 2% of the initial population had died, 92.2% of the alive population responded to a short questionnaire and 77.1% to a long questionnaire. In the third survey (EGEA3), which was conducted between 2011 and 2013, 1558 participants returned their questionnaire (response rate = 79.2%).
A fourth survey (EGEA4), including a clinical examination will start in 2023.
A better understanding of the relationship between asthma and environment, lifestyle factors and genetics is important to develop preventive measures and to improve the asthma management of patients. The EGEA study provides public health information with the objective of improving the respiratory health at the population level.
Who participates in the study
In the first phase of the EGEA study (EGEA1), 388 asthma families and 415 participants from the general population were included, representing 2047 participants with or without asthma.

Family type 1
Family type 2
388 families included from a participant with the status of a child (offspring) in the family (family type 1) or by a participant with the status of a parent in the family (family type 2), for a total of 1632 participants.
In the second phase of the study, EGEA2, 73 new family members joined the study. A total of 2120 participants were involved in at least one phase of the study.

Control adult

Control child
415 participants from the general population (children or adults).